Research fields
Tumors arise and grow as a result of genetic changes that i. e. generate oncogenic proteins. The concept of personalized cancer therapy was developed to attack these factors specifically. However, intervention bychemical inhibitors harbors the risk of bypass reactions and the development of resistance mechanisms. We are therefore make use of principle of RNA interference (RNAi)-mediated inhibition of various oncogenes as a therapeutic option in tumor treatment and have developed an antibody-targeted nanocarrier system based on the natural protein protamine that specifically binds the RNAi-mediator siRNA and transports it safely to the target cancer cell.
Our research
Until now, the therapeutic use of RNAi has always been compromised by the missing delivery of the active substance siRNA to the target cell. For this purpose, we have developed a principle of tumor cell-specific application of siRNAs using target cell-specific surface receptor antibodies. The siRNAs are directed against highly specific oncogenes, and a combination of different siRNAs is also applicable. Here, the use of an oncogene-specific siRNA and the choice of a tumor-specific surface receptor offers a double level of specificity and focuses the siRNA treatment on the tumor cells. The universal carrier protamine has been known from clinical use for decades and can be used in all applications.
Our work is supported by the German Cancer Aid, the Wilhelm Sander Foundation, the German José Carreras Leukemia Foundation, the German Research Foundation, the ForTra gGmbH of the Else Kröner Fresenius Foundation, and funds given by the Medical Faculty of Münster.
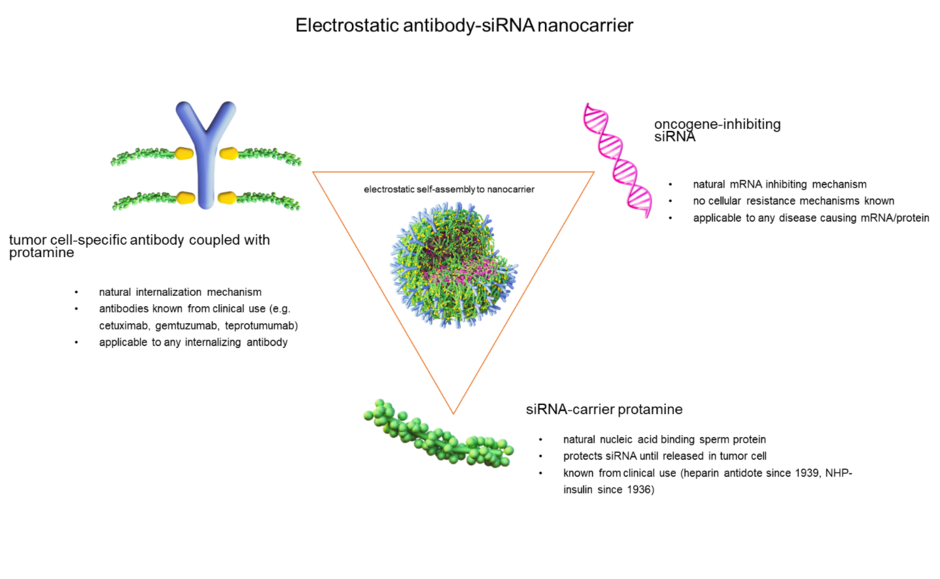
In our concept of siRNA therapy, stabilized nanocarriers for the treatment of tumor diseases are produced by electrostatic interactions. For this purpose, target cell-specific antibodies of our choice are chemically conjugated with protamine and assembled along with free protamine and siRNA to form a spheroidal nanocarrier. The process follows an auto-assembly principle, with the components self-organizing into a structure that is optimally balanced. The nanocarriers are decorated with the antibodies and contain siRNA that is protected within this vesicular structure.
Acute leukemias are malignant diseases of the hematopoietic system, which are characterized by an uncontrolled proliferation of immature progenitor blood cells, the so-called blasts, in the bone marrow. Depending on which type of blood cell is affected, a distinction is made between acute myeloid leukemia (AML) and acute lymphocytic leukemia (ALL).
Recently, mutations in the methylating enzyme DNA methyltransferase 3A (DNMT3A) have been found in up to 20–30% of all AML patients. These mutations can even be detected in early bone marrow cells before leukemia develops. Thus, one can postulate that mutated DNMT3A paves the way to leukemia and is essential for the development of leukemia. Our working group has developed a system with which these siRNAs are bound to a protamine-nanocarrier that is decorated with the leukemia cell-specific anti-CD33 antibody so that the siRNAs are specifically transported to the leukemia cell in vivo and can be internalised into the cell protected from degradation. We found that inhibition of DNMT3A via antibody-siRNA nanocarriers leads to reduced growth and increased cell death of leukemic tumor cells in vitro and in vivo in mouse models, indicating a therapeutic effect on the leukemia cells.
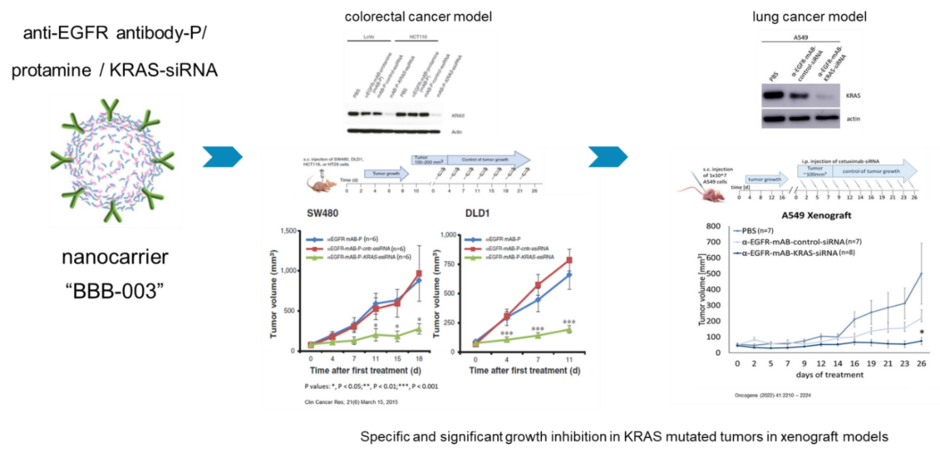
We here focus on solid tumor entities such as non-small cell lung carcinoma, colorectal carcinoma and pancreatic carcinoma. We combine a personalized therapy, i.e. therapy tailored to the tumors oncogenes, with the targeted application. We have developed a nanocarrier against the important cancer-causing factor KRAS for EGFR-positive tumor diseases, which, by means of its anti-EGFR - antibody equipment is taken up specifically via the EGF receptor in these tumor cells and thus causes growth inhibition and apoptosis via KRAS inhibition.

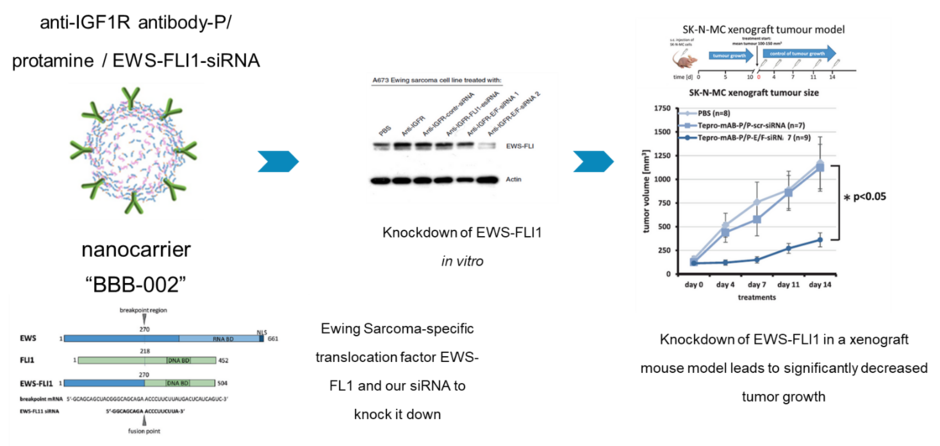
Ewing's sarcomas are an aggressive type of tumors that belongs to the rare diseases. About a third of patients with metastases die within the first five years after diagnosis. The genetic causes for the growth of an Ewings sarcoma are well known: There is a genetic change that causes the formation of a new oncogenic factor, the so-called fusion protein EWS-FLI1. This changes the cells so that they grow uninhibited. This factor and of Ewing's tumor cells have already been inhibited in the laboratory. This did not work in the patient, the EWS-FLI1 factor was considered “undruggable”.
We established a nanocarrier made of protamine, a protein that is produced naturally in the body, which protects the siRNA from degradation and decorated with antibodies against the IGF1-receptor capable of detecting and entering Ewing's tumor cells.
As a result, the nanocarriers formed by IGF1R-antibodies, along with the protamine and the siRNAs against the EWS-FLI1 bind to the Ewing cells and are internalized. Inside the cell, the siRNAs can then prevent the translation of the EWS-FLI1 oncoprotein and thus deprive the Ewing tumor cells of the basis for their uninhibited growth
Team leaders

Dr. rer. nat. Sebastian Bäumer
Wissenschaftlicher Mitarbeiter

Dr. rer. nat. Nicole Bäumer
Wissenschaftliche Mitarbeiterin
Click here for the german version.
Our team
Team leaders

Dr. rer. nat. Sebastian Bäumer

Dr. rer. nat. Nicole Bäumer
Senior Professor

Univ.-Prof. Dr. med. Wolfgang E. Berdel
PhD-student

Katharina Grunert
MD-student
Najeeb Shammaa
Technicians

Mara Apel

Lisa Wittmann
Medizinische Klinik A (Hämatologie, Hämostaseologie, Onkologie und Pneumologie)
Albert-Schweitzer-Campus 1
Gebäude A1
48149 Münster

